- 작성일
- 2018.09.27
- 수정일
- 2018.09.27
- 작성자
- 총관리자
- 조회수
- 399
제 4 장 - 알켄(Alkene)의 반응
Reaction Types
additions
A + B --> C
H-Cl + CH2=CH2 --> CH3-CH2-Cl
eliminations
X --> Y + Z
CH3-CH2-Cl --> CH2=CH2 + H-Cl
substitutions
A-B + C-D --> A-C + B-D
CH4 + Cl2 --> CH3-Cl + H-Cl
rearrangements
X --> Y
cyclopropane --> propene
Reaction Mechanisms
a step-by-step account of how a reaction occurs (and why)
bond-breaking steps:
homolytic: one electron to each fragment
(generates radicals)
heterolytic: both electrons to one fragment
(generates ions)
bond-making steps:
homogenic or heterogenic
Electron Movement
electron-pushing arrows indicate the flow of electrons in a mechanism
electrophile:
electron-deficient species seeking a pair of electrons (a Lewis acid )
nucleophile:
electron-rich species that can provide a pair of electrons (a Lewis base)
HCl plus Ethene
CH2=CH2 + H-Cl --> CH3-CH2-Cl
an electrophilic addition
reaction type: addition
reagent type: an electrophile
HCl, actually H+, a strong Lewis acid
Addition Mechanism
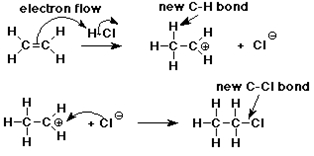
pi bond is relatively reactive, especially towards electrophiles
it provides a good source of electrons
addition of H+ to CH2=CH2 forms a new C-H sigma bond
the electrons for the new bond came from the pi bond
the other C is left with only 6 e-
Carbocation Intermediate
an intermediate is formed in the reaction mechanism
CH2=CH2 + H+ --> CH3-CH2+
carbocation: a carbon atom with only 3 bonds (6 e-) and a positive charge
structure: sp2 hybridized (trigonal)
Formation of Chloroethane
the reaction is completed as chloride anion (a nucleophile) adds to the carbocation (an electrophile)
CH3-CH2+ + Cl- ---> CH3-CH2-Cl
Equilibrium
for a general reaction:
A + B <==> C + D
equilibrium constant, K
K = [C] [D] / [A] [B]
favorable reactions have large K
unfavorable reactions have small K
Kinetics
rates of reaction
may or may not correlate with the favorability of the equilibrium
rate depends on the mechanism
is there a good way to get from reactants to products?
Heats of Reaction
delta H (enthalpy change)
delta H = H(products) - H(reactants)
exothermic means delta H <0 (negative)
reaction gives off heat
endothermic means delta H >0 (positive)
reaction absorbs heat
Potential Energy Diagrams
draw exothermic reactions downhill
draw endothermic reactions uphill
Activation Energy, Ea
there is usually an energy barrier between reactants and products
activation energy represents the highest amount of energy necessary while travelling along the minimum-energy (easiest) pathway from reactants to products
The Transition State
structure of the molecule(s) at the highest point along the reaction pathway
the stability of the transition state (relative to reactants) determines Ea (rate of reaction)
Alkene Addition Reactions
pi bonds undergo addition reactions
CH2=CH2 + HCl --> CH3CH2Cl
in general,
C=C + HX --> H-C-C-X
alkenes react with hydrogen halides to form alkyl halides
Addition of HX to Alkenes
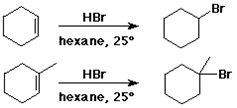
cyclohexene + HBr --> bromocyclohexane
1-methylcyclohexene + HBr --> 1-bromo-1-methylcyclohexane
(not 1-bromo-2-methylcyclohexane)
Reaction Notation
reactants -------> products
focus on the organic reactants and products
show reagents over the arrow
show solvent and conditions under the arrow
(or show full balanced reaction)
Orientation of Addition
regiochemistry:|
specific orientation of addition
(which C gets H, which gets X?)
alkene additions are regioselective:
one direction of addition is usually preferred
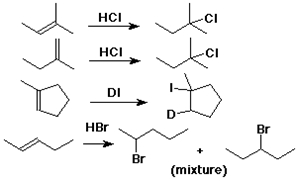
Markovnikov's Rule
the original:
add H to the C with more H's
(or to the C with fewer alkyl groups)
the reason:
add H+ to form the more stable cation
CH3CH=CH2 + HCl --->
CH3CH+CH3 (not CH3CH2CH2+)
---> CH3CHClCH3 (not CH3CH2CH2Cl)
Carbocations
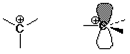
structure: trigonal (sp2)
stability: 3° >2° >1°
more alkyl groups stabilize a cation by electron donation to the electron-deficient (6-electron) carbocation
Markovnikov Addition
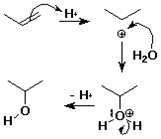
Hydration of Alkenes
alkene + water --> alcohol
CH2=CH2 + H2O --(H+)--> CH3CH2OH
mechanism:
step 1:
addition of H+ electrophile to pi bond
step 2:
addition of H2O nucleophile to cation
Hydration Mechanism
Halogenation of Alkenes
CH2=CH2 + Cl2 ---> Cl-CH2-CH2-Cl
mechanism:
Cl2 is an electrophile (adds Cl+)
then Cl- is a nucleophile
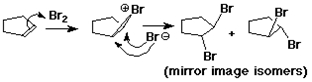
Anti Addition
anti stereochemistry:
two new groups are added to opposite sides of the original pi bond
cyclopentene + Br2 ---> trans-1,2-dibromocyclopentane (no cis)
anti - describes the process
trans - describes the product
Bromonium Ion
carbocations can be stabilized by bonding to a neighboring Br
(also works with Cl, but less favorable)
Reduction of Alkenes
reduction - addition of H2
(or removal of O)
CH2=CH2 + H2 ---> CH3-CH3
R-O-H + H2 ---> R-H + H2O
Catalytic Hydrogenation
CH2=CH2 + H2 ---> CH3-CH3
requires an active catalyst, typically Pt, Pd, Ni, PtO2
reaction occurs on the surface
both Hs are delivered to the same side of the pi bond
Syn Addition
syn stereochemistry: two new groups are added to the same side of the original pi bond
1,2-dimethylcyclohexene + H2 --(cat)-->cis-1,2-dimethylcyclohexane(no trans)
syn - describes the process
cis - describes the product
Oxidation of Alkenes
oxidation - addition of O
(or removal of H2)
RCH2OH ---> RCH=O ---> RCOOH
there are a wide variety of oxidizing agents:
O2, O3, KMnO4, CrO3, Na2Cr2O7
metals in high positive oxidation states
Hydroxylation
alkene + KMnO4 --(base)--> 1,2-diol
addition of two OH groups is syn
cyclopentene --> cis-1,2-cyclopentanediol
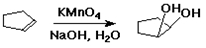
Oxidative Cleavage
C=C --> C=O + O=C
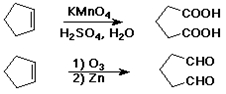
acidic KMnO4 causes cleavage
ozone (O3) causes cleavage
sometimes useful degradation method to identify unknown compounds
Balancing Redox Reactions
identify the two half-reactions
- what gets oxidized, what gets reduced?
balance all elements except O and H
balance O
use H2O (in acid) or OH- (in base)
balance H
use H+ (in acid) or H2O (in base)
balance charge with electrons (e-)
Polymers
long chains of repeating units (monomers)
n CH2=CH2 --(init)--> (init)-(CH2-CH2)n-
n=100-10,000 polyethylene
has properties like a very long alkane
many polyalkenes are commercially important materials and plastics
e.g., PVC, Teflon, Orlon
Chain Reactions
polymerization occurs by a free radical chain mechanism
initiation - generation of the first free radical from an initiator
R-O-O-R --(heat)--> 2 R-O·
(initiators have one weak bond)
Chain Reactions
propagation - radical adds to a p bond
RO· + CH2=CH2 ---> RO-CH2-CH2·
note that the product is also a radical
RO-CH2-CH2· + CH2=CH2 ---> RO-CH2-CH2-CH2-CH2· ---> etc.
typically this occurs hundreds or thousands of times
(until radicals recombine - termination)
Substituted Monomers
radical additions follow the Markovnikov Rule:
add radicals to form the more stable radical intermediate
radical stability is like cation stability: 3° >2° >1°
this leads to polymers with alternating substituents
Vinyl Polymers
polyvinyl chloride
polypropylene
polystyrene
Alkyne Additions
similar to alkenes but more reactive
Markovnikov Rule is followed
excess reagent gives double addition
single addition is usually possible
single addition gives alkene product, which may be cis (syn addition) or trans (anti addition) or nonspecific
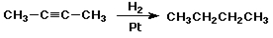
Reduction of Alkynes
excess H2 + catalyst gives alkanes
- 첨부파일
- 첨부파일이(가) 없습니다.